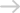
Harford, P.C., is now accepting cases on behalf of individuals who suffered lower extremity (leg, foot, and toe) amputations after taking the pharmaceutical prescription medication Invokana (Canagliflozin). The manufacturer of the Invokana was recently required by the U.S. Food and Drug Administration (“FDA”) to add these injuries within its most prominent portion of its label—the Boxed Warnings.
What is Invokana?
Invokana is a prescription drug developed, manufactured and marketed by Johnson & Johnson and Janssen Pharmaceuticals, Inc. to treat type 2 diabetes. It was initially approved by the FDA in January 2014 and is in a class of new diabetes drugs called glucose contransporter-2 (“SGLT-2) inhibitors. SGLT-2 is a protein in humans that facilitates glucose reabsorption in the kidneys.
As the name suggests, SGLT-2 inhibitors decrease sugar in the bloodstream by inhibiting glucose reabsorption. The extra sugar is then eliminated from the body through urine produced by the patient’s kidneys, putting extra strain on the kidneys of patients that already have increased insult by virtue of the patient having diabetes.
What are the risks of Amputations?
 On May 16, 2017, the FDA issued a safety communication confirming that Invokana use increases the risk of leg, foot, and toe amputations nearly two-fold, based on data from a study arising from two large clinical trials. The results from this large study (which incidentally was sponsored by the manufacturers of the drug) were published in the New England Journal of Medicine. The study, which was identified by the authors as the “CANVAS Program,” integrated data from a total of 10,142 participants with type 2 diabetes and high cardiovascular risk.
On May 16, 2017, the FDA issued a safety communication confirming that Invokana use increases the risk of leg, foot, and toe amputations nearly two-fold, based on data from a study arising from two large clinical trials. The results from this large study (which incidentally was sponsored by the manufacturers of the drug) were published in the New England Journal of Medicine. The study, which was identified by the authors as the “CANVAS Program,” integrated data from a total of 10,142 participants with type 2 diabetes and high cardiovascular risk.
CANVAS concluded that the risk of lower limb amputations was 5.9 amputations per 1,000 patients per year for Invokana (Canagliflozin) compared to 2.8 amputations per 1,000 patients per year for placebo. This represented a substantial risk of amputations for users of the drug. Despite the fact that Johnson & Johnson and Janssen sponsored and supported CANVAS, and received and were aware of the data before the publication date, they failed to make any changes to their label to alert physicians and patients about the risk. It was only until the FDA safety communication was released that any action was taken to change the label.
What is the status of any Litigation concerning these Injuries?
In December 2016, the U.S. Judicial Panel on Multidistrict Litigation ordered Invokana cases consolidated into a Multi-District Litigation in the United States District Court, District of New Jersey (MDL 2750). The cases allege that Johnson & Johnson and Janssen failed to conduct proper pre-market safety testing for Invokana, concealed the risks of lower extremity amputations, and refused to properly warn doctors and patients of these risks.
How can Harford, P.C., Help?
Harford, P.C., is currently accepting leg, foot, and toe amputation cases arising from the use of Invokana. If you or someone you know has been injured from the use of Invokana, please contact us immediately at (212) 390-8983 for a free case consultation. You may also complete the form on the side menu and we will contact you to set up a free consultation.
We are here to help you.