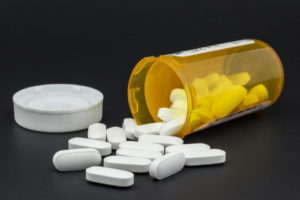
Harford, P.C., has been retained today by a client permanently injured in both lower extremities after using the pharmaceutical product Invokana—a prescription drug for patients with type II diabetes mellitus. The drug is manufactured and distributed by Johnson & Johnson and Janssen Pharmaceuticals, Inc.
Invokana, also known as canagliflozin, is a member of gliflozin class of pharmaceuticals also known as sodium glucose co-transporter 2 (“SGLT2”) inhibitors. These drugs operate by blocking the reabsorption of glucose into the bloodstream by the SGLT2 receptor and instead dumping the glucose through the kidneys and excreting it through the urinary tract. In patients with type II diabetes, the body produces insulin but does not produce enough and therefore cannot properly metabolize glucose for energy.
On May 16, 2017, the FDA released a Drug Safety Communication that canagliflozin causes “an increased risk of leg and foot amputations” based on new data from two large clinical trials. The FDA required the manufacturer to place new warnings within the drug label’s most prominent Boxed Warnings to alert physicians and patients of the risk of amputation. As of July 2017, the black box warning within the labels of Invokana, Invokamet and Invokamet XR (the latter two being combination drugs of Invokana and Metformin) now notifies physicians of the risk of lower limb amputations from taking these drugs.
After taking Invokana to treat type II diabetes, our client initially experienced severe pain and internal wounds in his lower extremities and feet. He thereafter underwent multiple vascular surgeries to avoid amputations in both feet, is now unable to walk without assistance, experiences constant sharp pain in his feet, and lives everyday with the fear that his feet will be amputated.
Lawsuits against Johnson & Johnson and Janssen over the drug Invokana, and its sister canagliflozin drugs, have been consolidated for pretrial proceedings in the United States District Court in New Jersey before the Hon. Brian R. Martinotti in Trenton, New Jersey.
The litigation is entitled In re: Invokana (Canagliflozin) Products Liability Litigation, MDL No. 2750.
Harford, P.C., is currently accepting leg, foot, and toe amputation cases arising from the use of Invokana. If you or someone you know has been injured from the use of Invokana, please contact us immediately at (212) 390-8983 for a free case consultation. You may also complete the form on the side menu and we will contact you to set up a free consultation.
We are here to help you.